

Refer to Appendix A for a more complete activity series of metals and of halogens.
Halogen activity series how to#
ĭescribe how to use the activity series of metals and the activity series of halogens. If you predict that there will be no reaction, write NR. Using the activity series for halogens, write a balanced chemical equation for each single displacement reaction. So, given the reactants fluorine and sodium chloride, you can predict that the following reaction will occur. For example, fluorine is above chlorine in the activity series. In the same way as you used the activity series for metals, you can use the activity series for halogens to predict whether substances will undergo a single displacement reaction. Fluorine is the most reactive, and iodine is the least reactive. It can be shown simply in the following way. The activity series for halogens directly mirrors the position of halogens in the periodic table.

For example, a series of bromoperoxidases have been isolated recently from seaweed17,18 and an actinomycete19 that require vanadium for halogenating activity. There are several examples of haloperoxidases containing metals other than iron, and many of these are non- heme enzymes. Therefore, a separate activity series for the halogens can be developed. Halogen reactivity decreases as one goes from top to bottom in the periodic table, because of the decreasing electronegativity. In the second reaction, chlorine is more reactive than bromine because bromine is listed below chlorine in the halogen activity series.
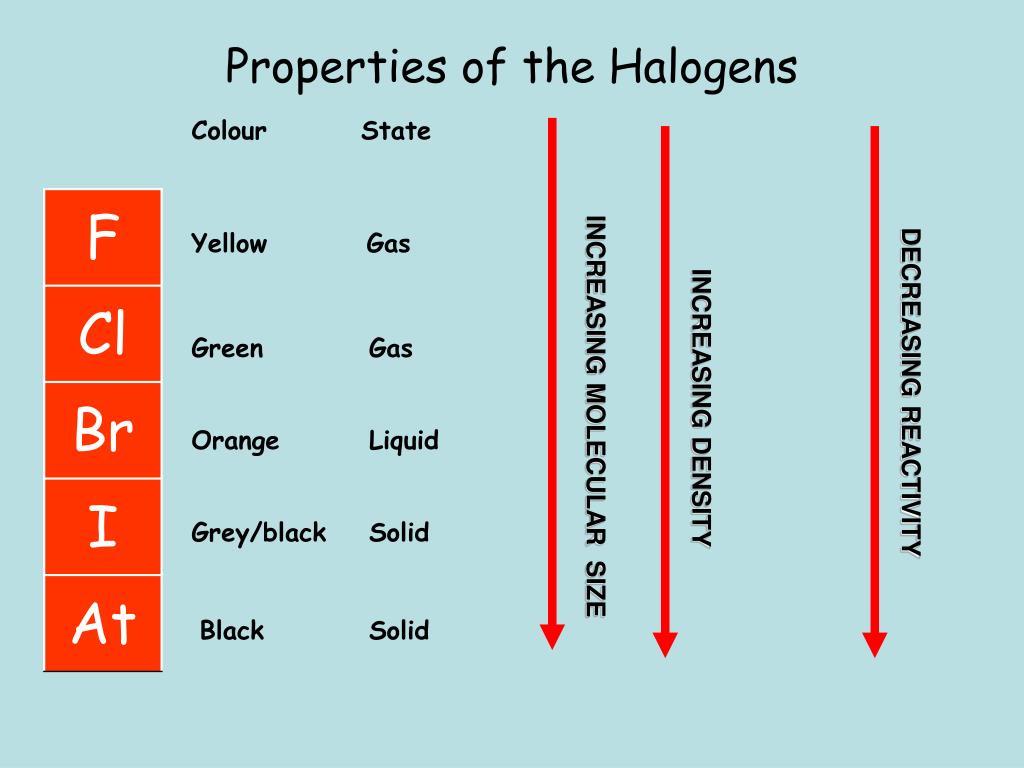
īased on what you know about the electronegativity and electron affinity for the halogens, explain the organization of the halogen activity series. Try the following problems to practise using the metal and halogen activity series to predict whether reactions will occur.


 0 kommentar(er)
0 kommentar(er)
